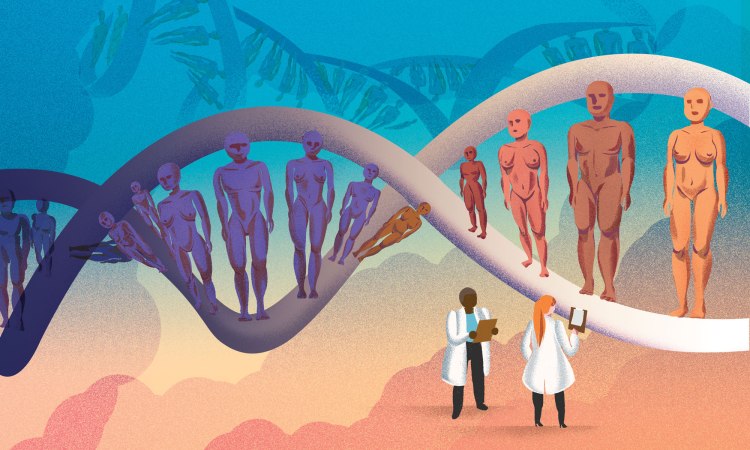
Indigenous biomedical researcher Keolu Fox makes the case for studying Indigenous people’s DNA, something that could yield benefits for all of humanity.
There’s a boxed warning that comes along with Plavix, the anti-blood-clotting drug that hit the market in 1997. If you’re a “poor metabolizer,” it warns, the drug may not be effective for you and might even lead to negative effects. How do you find out if you’re a poor metabolizer? You take a genetic test.
However, this FDA-mandated warning about genetic difference was added only in 2010 — after the drug had been on the market for more than a decade. In 2014, the state of Hawaii filed suit against Plavix’s manufacturer, Bristol-Myers-Squibb, because the genetic difference had been shown to have a larger-than-expected effect on two groups of people in that state. As the lawsuit said, “38–79 percent of Pacific Islanders and 40–50 percent of East Asians may respond poorly to Plavix due to a genetic predisposition to poorly metabolize the drug.”
How did a drug like this get through the American drug testing process, while being so ineffective for two large groups of people in the US? Likely because it was tested — as are 95 percent of all drugs — only on people of European descent. Stories like this are enraging, but they point to some intriguing possibilities, too. What if we treated humanity’s genetic differences not as a bug, but as a feature? And what if we looked closely at people with very distinct genomes to learn more about why drugs and diseases work differently in them? As an Indigenous scientist, I believe that paying attention to racial and ethnic diversity in biomedical research is not just a matter of social justice, it can also be a golden opportunity to understand more about disease.
Why do Indigenous people have genetic differences? Each of our genomes is composed of around six billion base pairs, but in terms of DNA sequence, every human is 99.5 percent similar to every other human. One significant way in which we diverge is in the diseases that are more common in certain ethnicities. A few examples: sickle cell anemia is found at a much higher frequency in individuals of African ancestry, thalassemia in individuals of Mediterranean ancestry, cystic fibrosis in individuals of European ancestry and Tay-Sachs disease among individuals of Jewish ancestry. These diseases are all monogenic, or caused by irregularities in a single gene occurring in all cells of a person’s body.
What we’re finding is, some of these diseases may represent adaptations to our ancestors’ local conditions. For instance, the sickle cell–shaped red blood cells found in communities in sub-Saharan Africa and the Philippines are a result of local adaption to the malaria parasite. Many Indigenous populations have been geographically isolated for tens of thousands of years, like my ancestors on the islands of Hawai’i. Over time, we have adapted to our environments in unique ways that leave signatures in our genomes, just like Darwin’s famous finches. We are a treasure trove of novel genetic variations.
There are some major challenges to studying Indigenous genomes. Indigenous people have historically opted out of being poked and prodded because, in the past, being part of medical experiments has not worked out well. In one egregious example, Havasupai tribe members provided genetic samples to Arizona State University scientists so they could study diabetes, but the researchers, without their subjects’ consent, used the DNA to look at schizophrenia, inbreeding and other conditions. Sometimes, Indigenous cultural values don’t allow for certain kinds of tests. For instance, scientists can use the sequence data generated from particular regions of the genome to trace a population’s migratory routes, giving them time estimates for migration and population divergence. However, many Indigenous groups do not want their origin stories challenged, so even though the scientific questions asked may be legitimate, researchers need to respect these wishes. Finally, some Indigenous people are less interested in participating in research because they have more basic concerns. When I gave a talk about preventive and predictive medicine at the National Congress for the American Indian in 2016, an elderly Native man came up to me afterward and asked point-blank, “Why should I care about genome sequencing? We haven’t had clean water on our land since 1912!”
Just as important as the social-justice need for broadening genetic testing is the idea that the biomedical community could be missing out on the most interesting genomes of all. Consider the Inuit, who have lived in the Arctic for thousands of years. They’ve adapted to the cold climate and a diet rich in fat from sea life, yet they have relatively low levels of heart disease and their genetic adaptations might unlock the secret to better heart health for everyone. Researchers believe they have identified mutations in a unique set of genes (FADS) that play a role in height, weight, fat distribution, and muscle and heart development. And if we can identify mutations in genes that play a key role in lowering cholesterol, for example, we might be able to use that information to start developing novel drug treatments.
In addition, diverse genes can help us understand the complicated way in which diseases work. In the current state of medical research, heritability appears to be king, but on closer look, the genetic contribution to disease becomes less clear. For instance, breast cancer can be broken into multiple types. Mutations found in the BRCA1 breast cancer gene have been extensively studied in woman of European ancestry, while it’s been shown that African American women are at a higher risk of developing triple-negative breast cancer (breast cancer that is estrogen receptor-negative, progesterone receptor-negative and HER2-negative). But are these two types of cancer susceptibility entirely genetic? What other factors — especially social determinants like diet, socioeconomic status and access to healthcare — come into play? By being able to separate the genetic contribution to diseases, we could start to tease out the non-heritable causes and figure out ways to counteract or minimize them.
The next few decades of research will be about sorting through the intertwined relationship between social determinants and the genetic predilection to disease. Scientists will need to design elegant experiments that determine which factors matter and then create precision treatments tailored to the genetics and realities of each individual. We must step away from the idea that European genes are the standard model. In doing so, we may see that the wonderful diversity of humanity — including our many kinds of Indigenous genes — could potentially hold the key to the next medical breakthrough to benefit all of us.