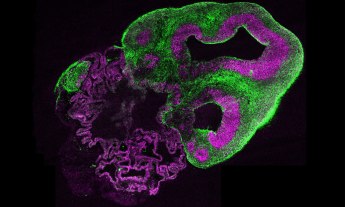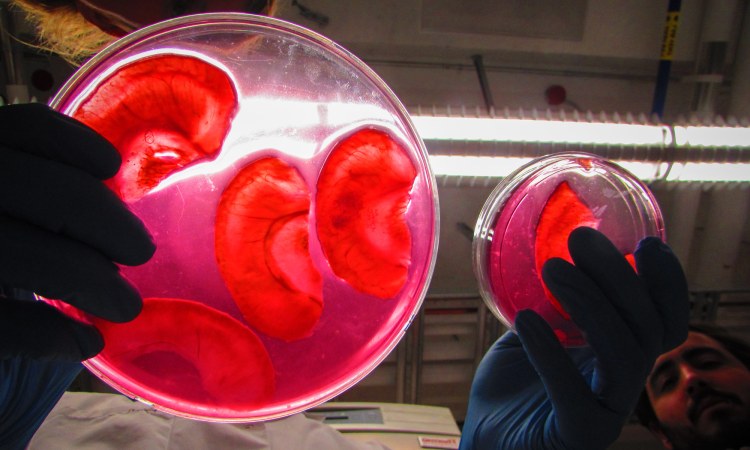
Biohacker and TED Fellow Andrew Pelling creates living, functional biological objects that don’t exist in nature — without deliberately modifying DNA in any way. In his lab at the University of Ottawa, he’s even figured out how to use apples and human cells to make ears in a petri dish. But how do you do that — and more importantly, why would you want to? The answer is surprisingly simple, and could mean greater access to medical innovation and well-being for all.
So. About those ears. Are they real human ears? Can they … hear? The answer is complex. Yes, the ears are made up of real, living human cells, but the material that gives them structure — the scaffolding — is apple cellulose. The ears were carved into the cellulose by hand (by Pelling’s wife, as it happens). Think topiary. And no, they can’t hear.
This may sound like just a creepy science art project, but it demonstrates an important point: that human cells can thrive on the fibrous structures of plants. And why is that important? Because it suggests the possibility of a low-cost, globally accessible biomaterial with which we might reconstruct our falling-apart bodies: skin, bones, veins, organs and so on.

Here’s how scientists are trying to make replacement body parts now. Lab-grown organs are at the frontier of medicine, with scientists experimenting with growing bone, cartilage, even more complex organs like kidneys and hearts. But all of them need a base material that can host living cells. “The common wisdom has been that scaffolds should be as native to the human body as possible,” says Pelling. There are several approaches for creating these scaffolds, but one promising approach uses the existing scaffold of a donated organ. In this case, scientists would remove the donor’s cells from the tissue, use what’s left behind as the protein scaffold, repopulate it with the patient’s stem cells and then transplant the organ back into the patient. (In a heart, what’s left behind when a donor’s cells are stripped out looks like a white ‘ghost heart,’ and is primarily made of collagen.) It can be a fiendishly complicated process, and so far scientists have only seen success with simpler organs such as bladders. “The jury is still out on whether it will actually work with an organ like the heart,” says Pelling.
Scientists have also spent decades trying to engineer synthetic scaffolds so that blood vessels will grow inside of them. This too can be complex and expensive. “The equivalent amount of synthetic commercial biomaterial needed to create an organ can sometimes cost thousands of dollars on the market,” Pelling says.
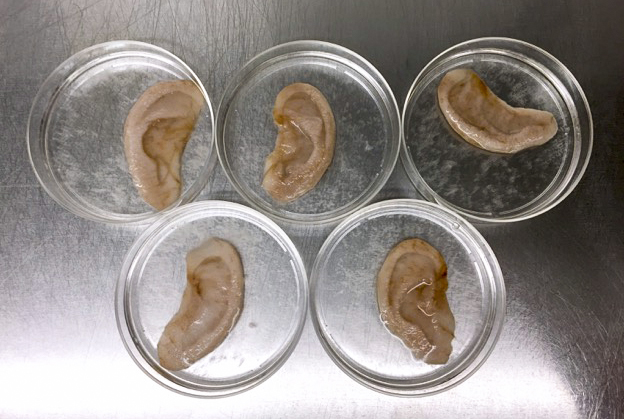
Apples? Apples grow on trees. Apples are cheap. Whereas previous attempts had used synthetic or highly processed natural cellulose, Pelling’s lab had the idea to use plant cellulose straight from the apple, with very few processing steps and as simply as possible. “Our hypothesis was that apple cellulose would act just like any other scaffolding,” says Pelling, who runs the publicly funded Pelling Laboratory for Biophysical Manipulation at the University of Ottawa, and who has a long interest in experimental biophysics, as this field is known. “To get to the cellulose, PhD student Daniel Modulevsky devised a protocol in which you slice an apple, wash it in soap and water, then sterilize it. What’s left is a fine mesh of cellulose into which you can inject human cells — and they grow.” says Pelling. The next logical question: can you implant it? The answer, Pelling found, is yes. “Modulevsky then collaborated with senior lab scientist Charles Cuerrier and discovered that when you implant it under skin, the surrounding cells enter the mesh and send out signals to create a blood supply, and it becomes a living part of the body.”
The method goes beyond ears on apples. Asparagus may one day help repair spines. Researchers are now plowing through other vegetables to examine their possibly useful properties. “The lab sometimes looks like a farmer’s market when we stock up on supplies, because we’ve been exploring every single plant we can get our hands on,” says Pelling. There are many interesting structures already in nature that might be used as possible scaffolds for repairing various parts of the human body. “Some plants have three-dimensional structures that are really similar to existing commercial synthetic scaffolds,” he says. But if there are commercial products available, why tinker with plants? Well, he asks: “If it’s easy to produce and nature has already done the work for us, why not?” The Pelling Lab is now examining more candidate plants such as pears, asparagus and mushrooms for their potential in repairing bone, nerves and skin.
A novel approach to innovation: “bonkers experimentation.” Pelling’s lab didn’t set out to create a new way to make materials for tissue engineering. “We were actually joking around about making that monster plant Audrey II in Little Shop of Horrors,” says Pelling. Which brings us to the value of play. Pelling describes his lab as a fundamentally playful, exploratory space where scientists, engineers and artists gather to ask questions and try to figure out how to answer them, with no attachment to outcome. “It’s just an environment where people can be curious,” he says. “We don’t deliberately embark on doing research with real-world applications.”
In fact, it was a rummage through dumpsters to find hardware to disassemble and reassemble (just for fun) that got Pelling wondering whether he could do a similar process with a living system. Back in the lab, that idea morphed into the apple experiment. “To our surprise, it turned out to be far easier than we thought it would be, and after some optimization it eventually worked,” he says, adding that it might not have happened at all if he’d been thinking conventionally. “We kind of took a pretty Stone Age approach, to be honest.”

It’s not genetically modified, and it’s not synthetic biology. So what’s it called? “When you say, ‘Oh, I’ve made an apple with human cells in it,’ people immediately think of genetic modification,” says Pelling. “But we don’t touch the genome. In fact, what makes this interesting is that we’ve transformed biological function without manipulating DNA code.” So how should we think of this field? “I’ve been calling it ‘augmented biology,’” he says. “Really, it’s a progression on an old idea. We already augment our bodies: tattoos, piercings, fillings.” Still, Pelling’s idea is unusual enough to make it sometimes challenging to find funding. So far, seed grants have allowed him to conduct preclinical research by testing out the safety and compatibility of the implants in mice.
“Once people get over the fact that it’s an implant derived from an apple, they realize we’re not doing anything incredibly different from current technologies,” says Pelling. “We’re just doing it cheaply and easily, and we’ve eliminated several barriers so it might actually have real impact. Now that we’ve come this far, I’m really curious to know what is, and what is not, actually possible.”
Perhaps you, too, might begin sculpting organs in your kitchen. Pelling feels strongly that scientific research and innovation should be open to anyone. “It really bothers me that scientific equipment can be very expensive, sometimes creating inequitable access to research-grade materials and hardware,” he says. “I’m always thinking about how we can do complex science simply and cheaply — when it’s possible.”
To that end, Pelling, Modulevsky and Cuerrier co-founded a company, Spiderwort, to sell kits for the hardware required to make cellulose-based biomaterials and the incubators required for growing human and animal cells. “The incubators are essentially warm boxes, 37 degrees Celsius, that control the carbon dioxide content in the atmosphere,” he says. Pelling designed the incubator himself and built the first ones primarily from components he found in the garbage. He released the instructions as open source, but subsequently received requests from the bio-art and scientific communities for kits. “Nobody wanted to go find materials in the garbage,” he says, “so we created a kit, and now it’s being beta tested.” The company is working on a larger open-source platform for biological hardware and wetware, aiming to enable everyone from DIY citizen-science labs, academic labs and even hospitals to create these biomaterials.

“I’ll take a curly one, please.” Beyond the pragmatic, Pelling is envisioning artistic as well as medical augmentation. “Moulded and 3D-printed organs are all well and good,” he says,“but if they are mass manufactured there is a possibility every organ in a batch will look really similar. I like the idea of our organs expressing some individuality. Maybe you’d carve your own ear, or commission an artist to create one, and not use a generic implant from a company. It’s interesting to think about how people might be able to take ownership of what their organs and implants look like, and maybe even how their bodies function.”